Product successfully added to your shopping cart
Quantity
Total
There are 0 items in your cart. There is 1 item in your cart.
Total products
Total shipping Free shipping!
Total
- Wechat ID: lcj391509517 Email: info@nbearth-star.com
products
New products
-

Flame Safety Valve with Bracket
Model.:SMT-FM009
-

5/8- 18NUF 8MM Shaft Brass Valve
Model.:SMT-FM009G
-

Patio Heater brass valve 2 Way
Model.:SMT-FM009F
-

Outdoor Patio Heater gas valve (elbow with nozzle)
Model.:SMT-FM009L-1/WTJ001
-
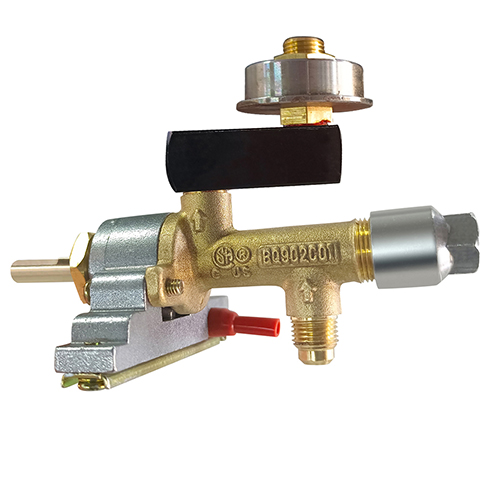
Copper valve inlet and outlet thread 7/16-24
Model.:SMT-FM009L
-
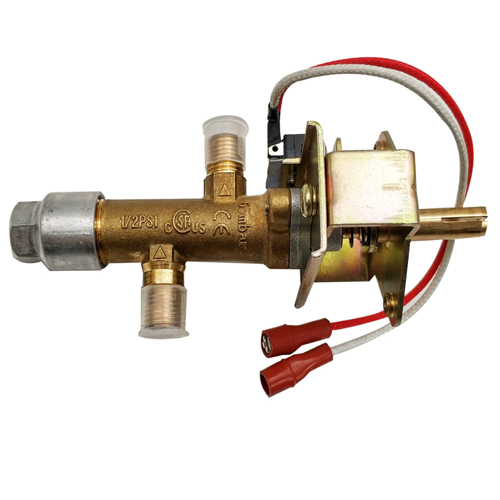
micro switch type gas valve for heater
Model.:SMT-FM008
News Center
How LPG Work
Date:2016/11/5
Description

LPG may be liquefied by moderately increasing the pressure or by reducing the temperature.
Refrigerated storage is often used by gas suppliers to store large volumes of LPG. The main form of LPG storage is in special containers known as 'pressure vessels'. Commonly these may be found as either 'bulk tanks' or ‘cylinders’. Because LPG has a high coefficient of expansion in its liquid form the tanks are never completely filled with liquid but to approximately 87% of their water capacity, the remaining 'ullage' space (i.e. The space above the liquid fuel) being taken up with vapour (often referred to as the ‘vapour space’ ) which allows the gas to be drawn off safely without any liquid carrying over into the pipework.
As gas (vapour) is drawn from the tank, the vapour pressure in the tank falls and the liquid boils, producing more vapour and restoring the pressure.To maintain boiling, the liquid absorbs heat from itself, from the metal walls of the tank in contact with the liquid (known as the wetted surface area) and from the air surrounding the tank. The available gas 'off take', therefore, is dependent upon the surface area of the tank, the quantity of liquid within the tank and the temperature. The low temperature of the liquid (often indicating excess ‘off take’) may be indicated as 'sweating' (where the water vapour in air condenses on the wetted surface area of the tank) and if the off take is large enough 'frosting' (where the condensed water vapour freezes) on the walls of the tank.
When the liquid temperature rises, for instance in summer, the vapour pressure increases, when the liquid temperature drops, the vapour pressure drops. Under normal UK conditions the pressure range will be between 2 to 9 bar.
